Abstract
BACKGROUND. We evaluated a human application of T cells genetically modified using the Sleeping Beauty (SB) transposon/transposase system to express a CD19-specific chimeric antigen receptor (CAR, designated CD19RCD28). The SB system uses a synthetic DNA transposon for nonviral somatic gene transfer. The initial data were reported (J Clin Invest. 2016 Sep 1;126(9):3363-76) and we now present long-term follow-up (LTFU).
METHODS. T cells were genetically modified using DNA plasmids from the SB platform to stably express CD19RCD28 and selectively propagated ex vivo with activating and propagating cells (AaPC) with cytokines. Twenty-six patients with advanced non-Hodgkin lymphoma (n=9) or acute lymphoblastic leukemia (n=17) underwent autologous (n=7) or allogeneic (n=19) HSCT followed by a T-cell infusion as adjuvant therapy on two clinical trials NCT00968760 and NCT01497184, respectively. Patients were subsequently enrolled onto a LTFU study, NCT01492036, to be followed for up to 15 years. During this time, patients could be tested annually for the persistence of genetically modified T cells using PCR and flow cytometry.
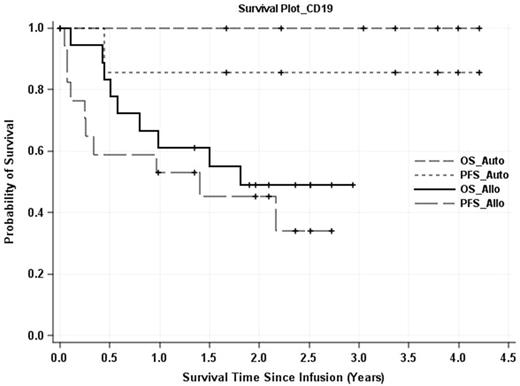
RESULTS. The persistence of SB-modified T cells was measured in two of the seven autologous HSCT/CAR+T patients as long as 4-years post infusion and both remain progression-free. Similarly, CAR expression was detected in three allogeneic HSCT recipients one-year post T-cell infusion with no disease progression. Upon LTFU of the autologous HSCT group, six of seven patients continue in complete remission (CR) at a median of 3.4 years (range: 1.7 to 4.2 years). The 3-year progression-free (PFS) and overall survival (OS) rates are 86% and 100%, respectively (Figure). After allogeneic HSCT, recipients received donor-derived CAR+ T cells (8 haploidentical, 10 matched-sibling, one 1Ag-mismatched sibling). For this allogeneic group, six patients remain alive and in CR following haplo (n=4) or matched sibling donor (n=2) HSCT with a median follow-up of 1.5 years (range: 0.003 to 2.9 years). The 2-year PFS and OS rates are 45% and 49%, respectively (Figure). Additionally, the 2-year PFS and OS rates for haplo-identical transplants were 57% and 71%, respectively. No long-term safety issues were identified; specifically, no graft failure or excess graft-versus-host-disease.
CONCLUSIONS. CD19-specific CAR+ T cells could be detected and were well-tolerated years after adoptive transfer and appear to provide long-term cancer control when infused after HSCT for patients with advanced disease. These encouraging results support further clinical development of this non-viral gene therapy approach.
Figure: Overall and progression-free survivals of recipients of SB-modified CAR+ T cells after autologous and allogeneic HSCT.
Huls: Former employee of Intrexon: Employment. De Groot: Ziopharm: Employment. Smith: Ziopharm: Employment. Yang: Ziopharm: Employment. Kantarjian: Bristol-Meyers Squibb: Research Funding; Novartis: Research Funding; Delta-Fly Pharma: Research Funding; Amgen: Research Funding; ARIAD: Research Funding; Pfizer: Research Funding. Cooper: Immatics: Equity Ownership, Patents & Royalties, Research Funding; Intrexon Corporation: Equity Ownership, Patents & Royalties; Sangamo Biosciences: Patents & Royalties; Organovo Holdings: Equity Ownership; Ziopharm: Employment, Equity Ownership, Research Funding; Procter & Gamble: Equity Ownership; Miltenyi Biotec: Honoraria; Ampliphi Biosciences: Equity Ownership; Argos Therapeutics: Equity Ownership; Ferring: Consultancy; Targazyme, Inc.: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal